Influence of Salinity on Surface Ice Adhesion Strength
Abstract
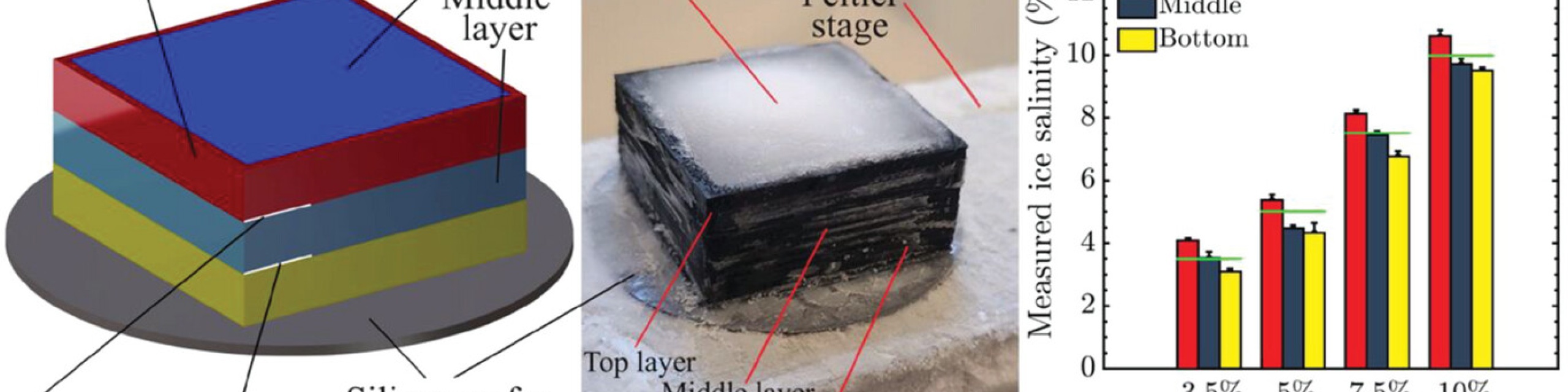
Ice accretion has detrimental effects on a wide range of sensitive engineering structures, especially those adjacent to or within the ocean. Here the effect of salinity on the adhesion of ice to different surfaces is investigated over a range of sub-zero temperatures. The saline ice adhesion strength is found to decrease with the increasing salinity on all surfaces tested. The presence of thermodynamically stable brine at temperatures above −21.2 °C is found to drastically lower the saline ice adhesion strength of essentially all surfaces through a lubrication effect. At −25 °C, below the eutectic temperature between H2O ice and hydrohalite (NaCl 2H2O), high adhesion is observed. For smooth surfaces, hydrophobicity is effective at lowering the saline ice adhesion strength, and a hydrophobic silicon wafer exhibited a saline ice adhesion strength of 2.4 ± 1.8 kPa at −10 °C with 1 wt% NaCl. The adhesion of real frozen seawater differed from the NaCl solutions, where an adhesion strength ≈12 kPa is observed even at −25 °C. Given the strong adhesion of ice to marine infrastructure, the results here demonstrate how temperature, salinity, and surface characteristics must be considered when designing materials that can mitigate the icing of marine infrastructure and vessels.
